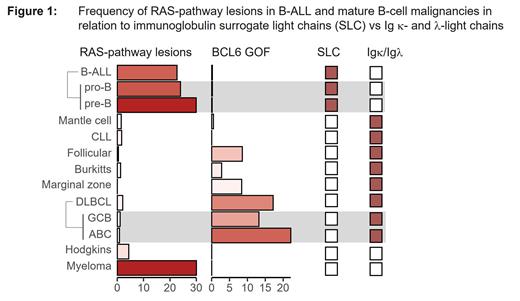
Background: RAS-pathway activating mutations (e.g. NRAS, KRAS, BRAF) cause constitutive ERK-activation which can lead to divergent outcomes. ERK-activation not only drives early stages of pre-B cell differentiation (Yasuda et al., 2008) but also induces negative selection and cell death to eliminate aberrantly activated B-cells at later stages of development (Limnander et al., 2011). Germline mutations in this pathway cause lymphoproliferative disorder and autoimmunity but not leukemia. Furthermore, activating lesions are very common in B-cell malignancies derived from the earliest (e.g. B-ALL) and the latest (e.g. multiple myeloma) stages of B-cell development, but they are rarely found in B-cell receptor (BCR)-expressing mature B-cell derived tumors ( Figure 1). These findings are consistent with previous observations that transgenic mouse models for RAS-oncogene were able to transform BCR-negative B-cell precursors to develop B-cell acute lymphoblastic leukemia (Chan et al., 2020). In contrast, transgenic expression of these oncogenes fails to transform BCR-positive mature B-cells (Mullins et al., 2013; Chung et al., 2014). Signals from the BCR, consisting of immunoglobulin (Ig) heavy chains (μ HC) and conventional light chains (kappa LC or lambda LC), and its precursor the pre-BCR, consisting of μ HC and surrogate light chains (SLC; λ5 and VpreB), regulate the development and function of B-cells. Here, we investigated whether structural elements of the (pre-) BCR determine the outcome of oncogenic RAS activation and how oncogenic RAS in B-ALL avoids cell death to promote leukemogenesis.
Results: We first studied the impact of oncogenic RAS-activation in BCR-positive mantle cell lymphoma cells. Expression of NRAS G12D in BCR-positive mantle cell lymphoma (JeKo-1) cells transiently reduced the surface expression of μ HC and eventually resulted in depletion of cells from culture in growth competition assays. We then examined whether CRISPR/Cas9-based genetic ablation of BCR components will enable permissiveness to oncogenic RAS signaling. Despite loss of μ HC function, oncogenic RAS caused depletion of JeKo-1 cells from culture. In contrast, this effect was partially reversed by genetic ablation of the constant region of immunoglobulin kappa LC ( IGKC).
We next examined whether reconstitution of surrogate light chain (SLC) components of the pre-BCR will confer permissiveness to oncogenic RAS signaling in JeKo-1 cells. We engineered JeKo-1 cells to express Dox-inducible expression of λ5 (Igll1) and VpreB. In the presence of kappa LC, overexpression of SLC alone did not reverse the deleterious impact of oncogenic RAS expression. Notably, reconstitution of SLC components following genetic deletion of IGKC amplified the effect of IGKC-deletion alone and largely rescued cells from oncogenic RAS-induced survival disadvantage. Taken together, our results showed that immunoglobulin light chains control the consequences of oncogenic RAS activation in B-cells - while SLC of the pre-BCR plays a productive role in enabling oncogenic RAS-signaling, conventional light chain of the BCR plays a counterproductive role.
Supporting this scenario, we found that SLC enabled oncogenic RAS to massively induce the proto-oncogene Bcl6 at the expense of the tumor suppressor Prdm1 to avoid cell death, promoting RAS-driven transformation of B-cell precursors. Upon genetic ablation of λ5, upregulation of Bcl6 was abrogated while Prdm1 was de-repressed. Consequently, RAS-driven malignant transformation of B-cell precursors was subverted. Reflecting the critical role of SLC in enabling RAS-BCL6 signaling, mature B-cells, which express BCR and lack SLC, failed to induce Bcl6 upon oncogenic RAS-activation, leading to upregulation of Prdm1. This observation may explain why persistent activation of the RAS-pathway in mature B-cells can lead to negative selection and cell death (Limnander et al., 2011).
Conclusions: Our results demonstrated that immunoglobulin light chains determine permissiveness to RAS-driven malignant transformation of B-cells. Expression of SLC enables RAS-BCL6 activation, allowing B-cell precursors to avoid PRDM1-mediated tumor suppression to promote leukemogenesis. In contrast, conventional LC opposes oncogenic RAS signaling, providing a mechanistic basis of the low frequency of RAS-pathway activating mutations in BCR-expressing mature B-cell lymphomas.
Disclosures
No relevant conflicts of interest to declare.